ABOUT EVENT
The Annual European Industry Touchpoint to Co-Develop
Drug-Diagnostics
As recent advances in small molecules, ADCs, cell, and gene therapies poise novel precision therapies from the bench to the clinic, the 15th World Clinical Biomarkers & Companion Diagnostics Summit Europe returned with 3 days of data-driven case studies and strategic insights to address your most prevalent pain points.
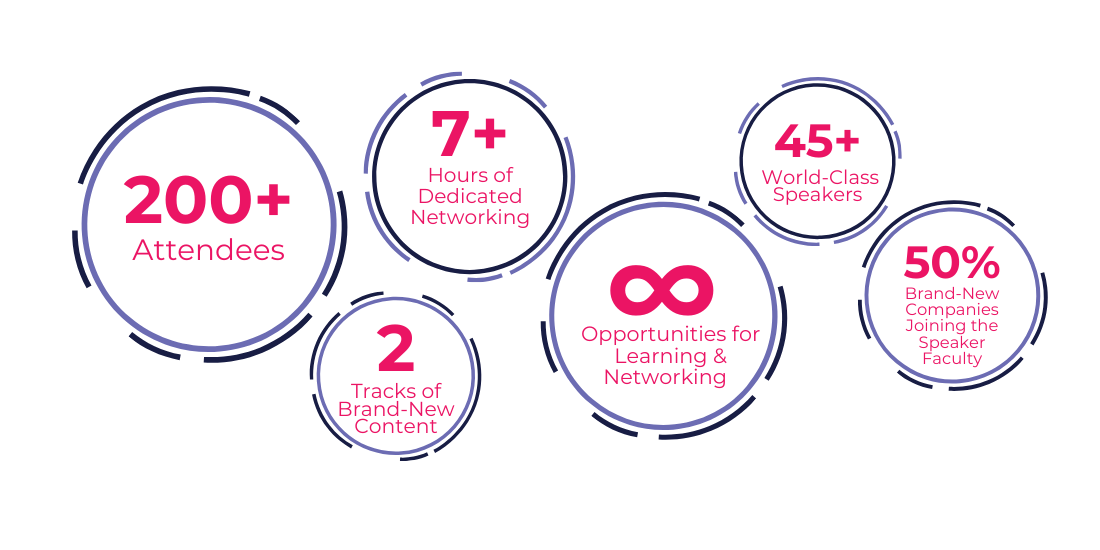
What You Missed:
What Your Peers Had to Say:
Amazing quality of speakers, underpinned by representation of different stakeholders from Pharma, IVD manufacturers and Notified Bodies, leading to valuable networking sessions.
- Past Attendee, Director

Great content, great networking opportunity and phenomenal industry experts!
- Past Event Partner, Marketing Manager

Interesting breadth of talks and panel discussions, accompanied by meaningful interactions with potential CDx company partners.
- Past Attendee, Director, Clinical Biomarkers