Partner With the Leading Drug-Diagnostic Forum Fuelling European Precision Medicine Collaborations
Europe’s precision medicine landscape is rapidly evolving, with biomarker strategies for next-generation therapies, the COMBINE pilot in action, and early detection initiatives scaling. To this end, Biopharma companies are forging strategic partnerships with diagnostics developers, testing laboratories, and regulatory experts to shape the future of drug-diagnostic co-development across oncology, neurological, rare, chronic, and other complex diseases.
Now in its sixteenth year, the 16th Clinical Biomarkers & Companion Diagnostics Summit Europe returns as the region’s most established meeting shaping biomarker strategy and day-one CDx readiness. Offering a critical window to establish or scale your presence in a market defined by fast-paced innovation, evolving regulation, and growing demand for localized and scalable diagnostic solutions.
Trusted by leading global drug developers and diagnostic innovators alike, this is where pivotal partnerships are formed, and next generation companion diagnostic and liquid biopsy platforms are accelerated for European market access.

What to Expect?
Meet New Clients Across Europe
Expand your European network with face-to-face engagement among senior precision medicine leaders from EU and UK biopharma, diagnostics developers, CROs, testing laboratories, and regulatory agencies, actively seeking innovative partners to accelerate biomarker discovery, CDx development, and IVDR-compliant clinical execution.
Centre Yourself in Biopharma Dealmaking
Strengthen your brand presence among senior industry decision-makers by showcasing your company logo on event materials including our website, brochure, and on-site signage.
Demonstrate Your Expertise
Present your services on centre stage, participate in a panel discussion or showcase your solutions in a bespoke exhibition booth to demonstrate your company as a trusted partner in advancing precision diagnostics and personalized therapy development across Europe.
Gain Market Intelligence
Stay ahead of rapidly evolving European market trends by hearing directly from industry pioneers and regulatory authorities. Understand competitor strategies, upcoming IVDR milestones, payer expectations, and emerging diagnostic models to refine your organization’s roadmap and sharpen your competitive edge.
Key Services & Solutions
Our industry attendees are looking for service and solution providers with capabilities in the below areas but not limited to:
Advancing compliant companion diagnostics solutions from bench to bedside
Improving early detection, patient selection and monitoring with liquid biopsy platforms
Identifying clinically meaningful biomarkers with novel IVD assay spanning imaging, functional assays, genetics, omics and multi-modal models
Enhancing data-driven strategies with AI-enabled translational tools and in vitro diagnostics
Bolstering accessible and scalable diagnostic testing across Europe through centralized and decentralized clinical laboratories
Accelerating towards harmonized Rx-Dx approvals, with strong IVDR experience and local medicines agency compliance
Integrating RWE with clinical trial results to inform iterative biomarker validation, label expansion, and adaptive trial design
Refining biomarker operations and pre-analytical workflows to ensure high-quality, reproducible biomarker data and workstreams
Hear What Our Past Sponsors Have to Say

The event was well organised, and a wide range of the audience. It was great to connect with people from Innovators to big industry leaders and pharma companies.
Panakeia

This is by far and away the best conference I’ve ever attended. The intimate sessions, positioning of the sponsor booths, frequent break times and partnering platform made this a great opportunity to network and learn.
Illumina

Great talks, relevant audience and as always, very well organised.
ANGLE

This European event had all the right people in one central place. Networking sessions were fantastic and talks well run.
ARC Regulatory
Audience Composition
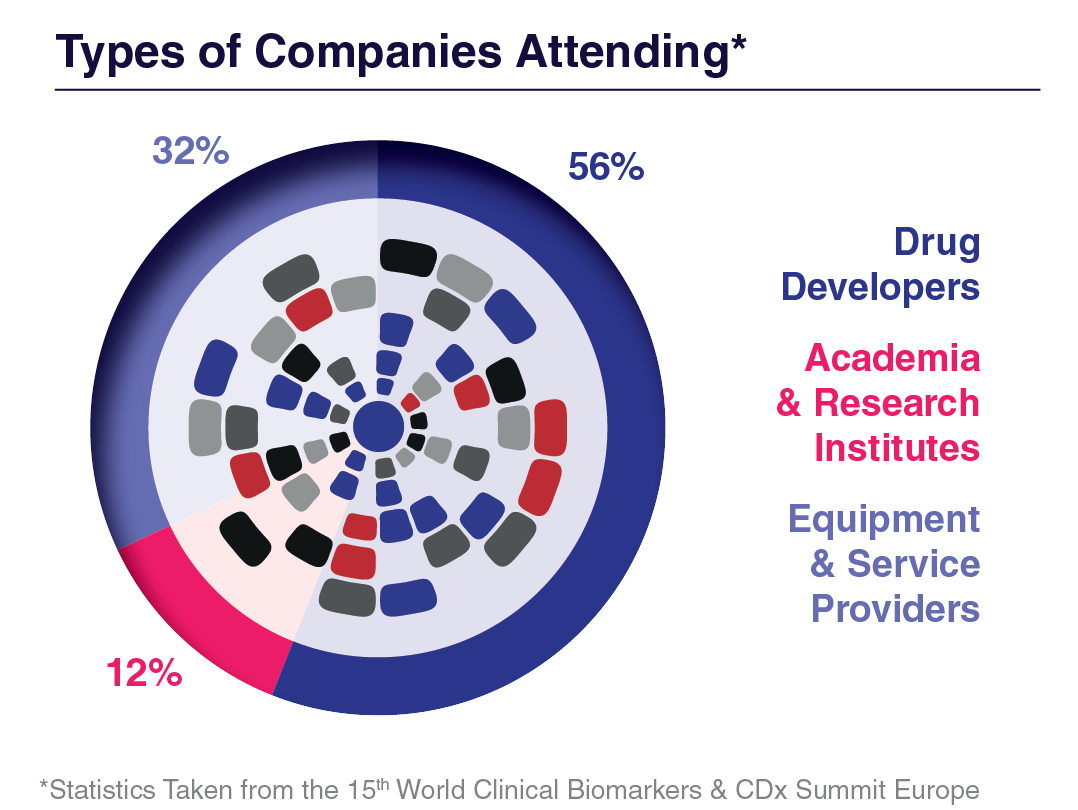
Company Type
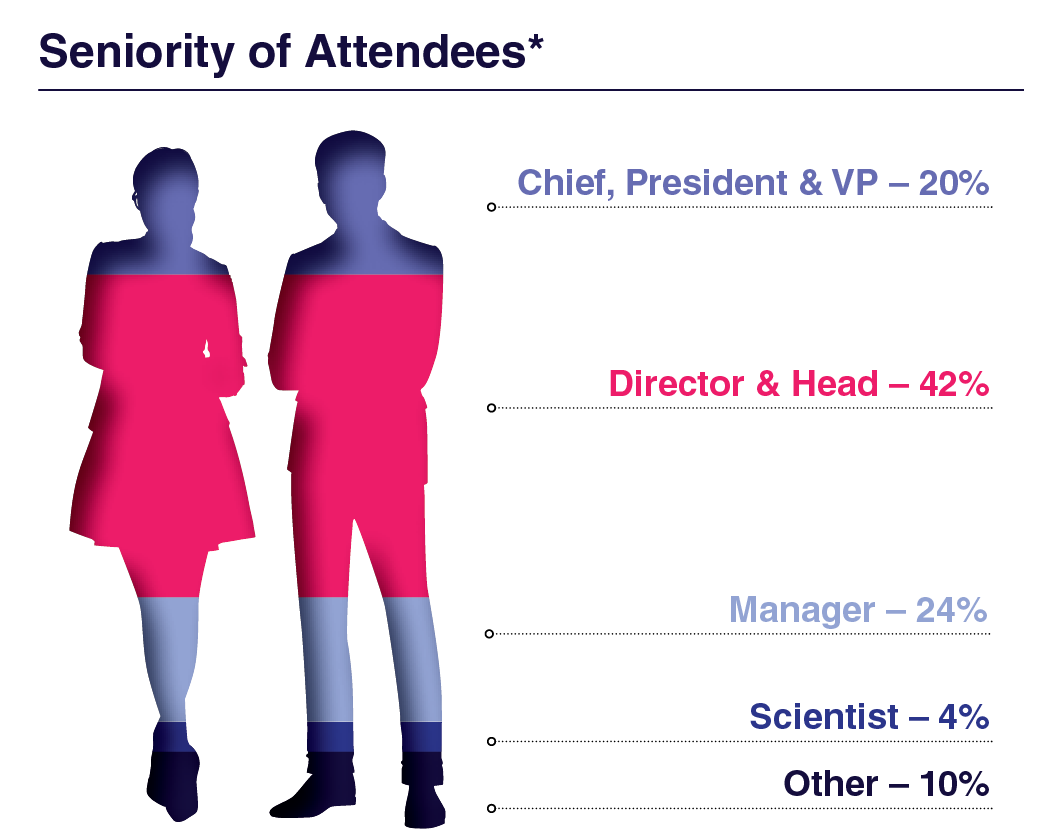
Attendee Seniority
Attending Companies Include














Get in Touch
Take advantage of our bespoke sponsorship opportunities to achieve your commercial goals. Email us if you would like to get involved and discuss a bespoke package suited to your needs.

Sam Sarwar
Senior Commercial Director



